What are the objectives?
We value the importance of evidence-based solutions to improve medication adherence. Therefore, we are currently conducting a clinical trial to test the effectiveness of the Collabree intervention among patients with hypertension in Switzerland. The study is designed as a randomized controlled interventional trial and is being conducted at the University Hospital Basel. We are recruiting men and women who suffer from high blood pressure and have been prescribed a therapy consisting of at least 4 tablets per day. Participants must also speak German or Swiss German and not be simultaneously participating in an interventional clinical trial. The goal of the study is to examine whether the Collabree application is effective in supporting patients with hypertension to follow their therapy plan more successfully. We will also investigate if the use of Collabree can help lower blood pressure.
How is the study designed?
The study consists of 4 in-hospital visits that take place during a 90-day adherence promotion program based on modern behavioral economics principles plus a 90-day follow-up period. During the visits, participants’ blood pressure is measured in the clinic as well as through 24-hour ambulatory blood pressure monitoring (ABPM), which is considered to be the gold standard for the diagnosis of hypertension [1] and for predicting cardiovascular outcomes[2]. Participants will also answer questionnaires regarding their self-assessed medication adherence and their experience with the mobile phone application.
Participants are randomly assigned to one of 3 groups in a ratio of 1:1:1. Two of these groups (Groups A and B) receive the Collabree mobile phone application with differing functions for each group (see table below for further descriptions of the study arms). Through the Collabree mobile phone application, participants will be reminded to take their medications based on their medication plan, and they will need to report their daily medication adherence. Participants in both the intervention groups as well as the control group will receive a smart dispenser for storing their antihypertensive medication. This dispenser records the day and time of openings, and these medication events will be used to validate the daily medication intakes that are reported in the mobile phone application. All participants receive standard of care. Please see the flow chart for an overview of the design.
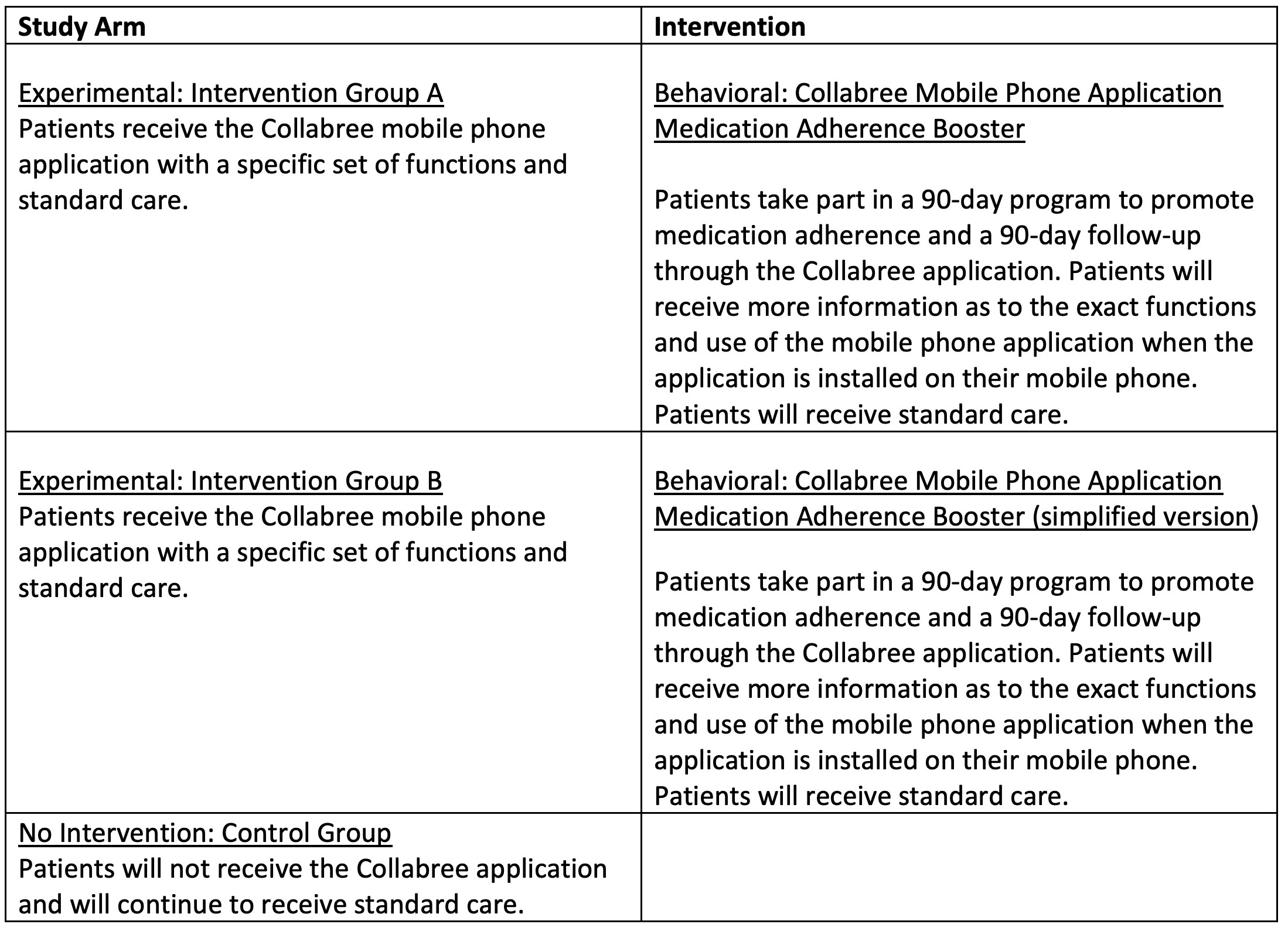 Table of details for each study arm in the clinical trial.
Table of details for each study arm in the clinical trial.
What outcomes are being measured?
We plan to recruit 180 participants in total, with 60 participants in each treatment group. The primary outcome measure is the change from baseline in medication adherence (monitored by medication events) at endpoint (i.e. after 90 days) for the intervention Group A relative to the control group. Secondary outcome measures include the change from baseline in the in-app medication adherence (defined as the number of medications self-reported as “taken” during the intervention period of divided by the number of prescribed medications listed in the in-app medication plan during a defined time period) at endpoint and follow-up (i.e. after 180 days) for the interventions Group A vs. Group B relative to the control group. The change in blood pressure will also be compared for the interventions Group A and Group B relative to the control group across time from baseline to follow-up.
This ongoing study has been approved by the Local Ethics Committee of Northwestern and Central Switzerland as a clinical trial.
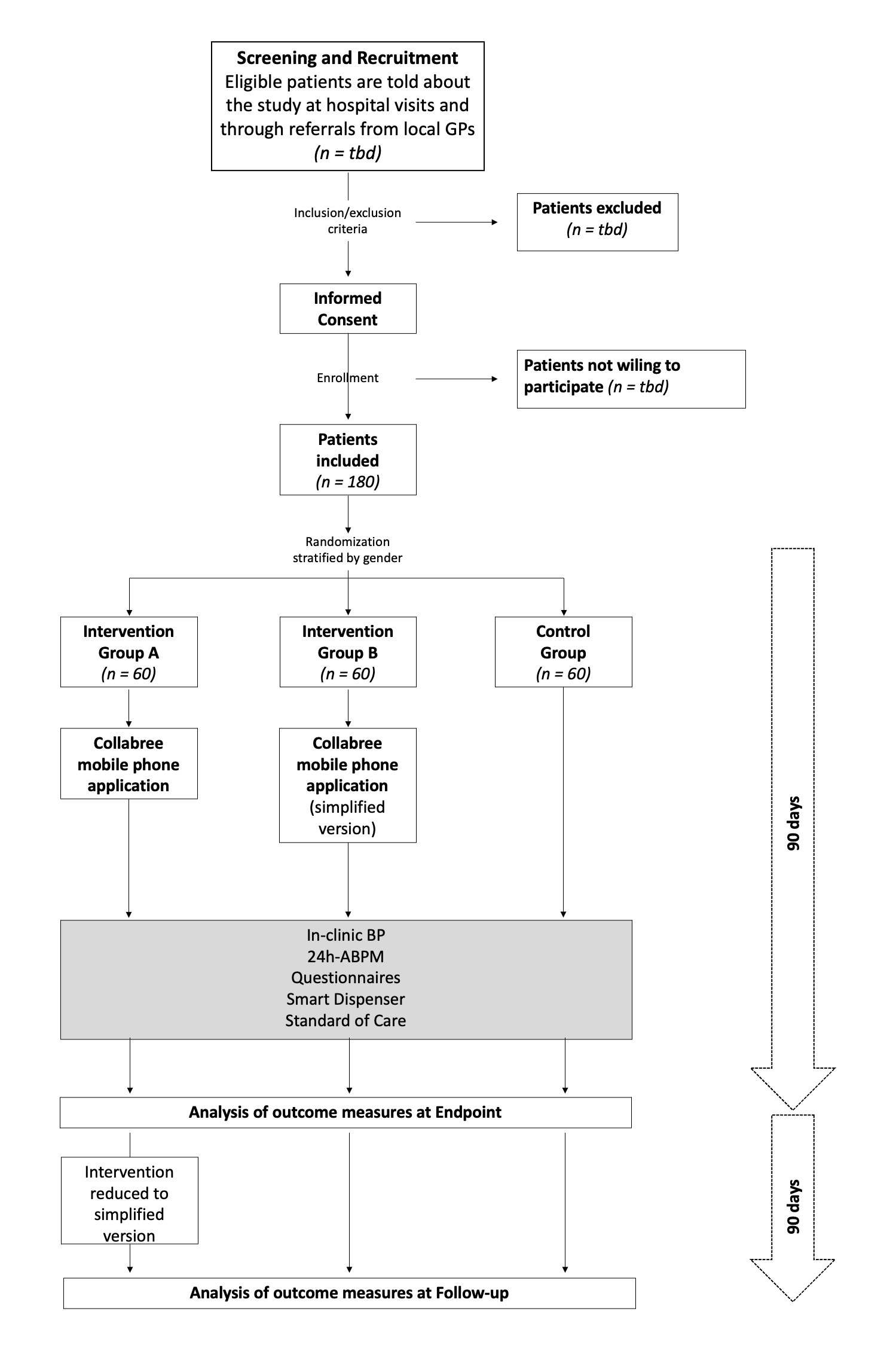 Flow chart of the clinical trial. BP, Blood Pressure; 24h-ABPM, 24-hour Ambulatory Blood Pressure Monitoring.
Flow chart of the clinical trial. BP, Blood Pressure; 24h-ABPM, 24-hour Ambulatory Blood Pressure Monitoring.
Find out more about our clinical trial
For more information about our clinical trial, please see:
ClinicalTrials.gov
Swiss National Clinical Trials Portal
or feel free to get in touch with us:
Dr. Anjali Raja Beharelle
CSO – Collabree AG
[email protected]
Pascal Kurz
CEO – Collabree AG
[email protected]
Interested in participating?
If you are interested in participating yourself or know someone who would be eligible, you or they can sign up here:
Literature
[1]: Hermida RC, Smolensky MH, Ayala DE, Portaluppi F. Ambulatory Blood Pressure Monitoring (ABPM) as the reference standard for diagnosis of hypertension and assessment of vascular risk in adults. Chronobiol Int. 2015;32(10):1329-42. doi: 10.3109/07420528.2015.1113804. [2]: Hodgkinson J, et al., Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ. 2011;342:d3621. dol: 10.1136/bmj.d3621.